1. OBJECTIVE To lay down a procedure for preparation, storage and use of culture media for...
Author
"A seasoned professional with a Master's in Chemistry, the author brings more than two decades of expertise in Quality Control, Analytical Method Validation, and advanced QMS investigation. Their experience is globally validated by successful management of major regulatory audits, including those conducted by the US, EU, Brazil, and Russian authorities."
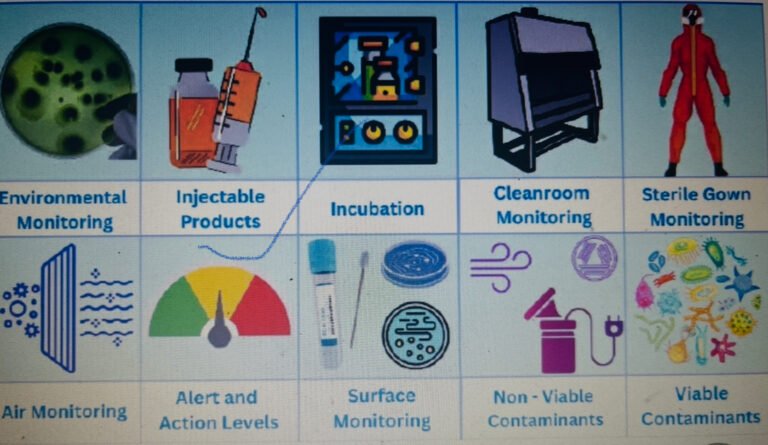
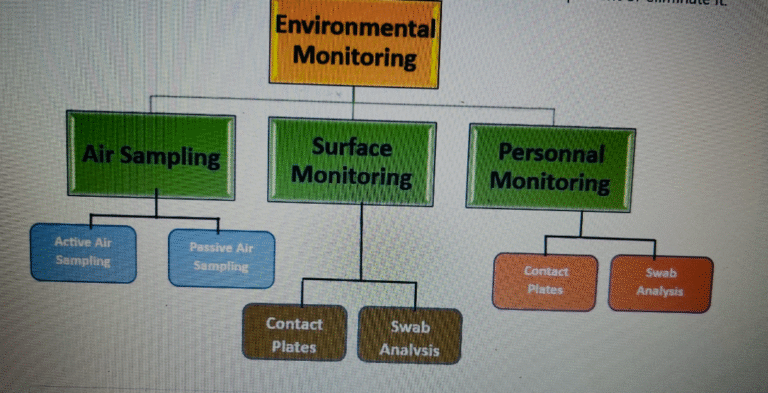
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
OBJECTIVE To lay down a procedure to provide general guideline to be followed in Quality control department...
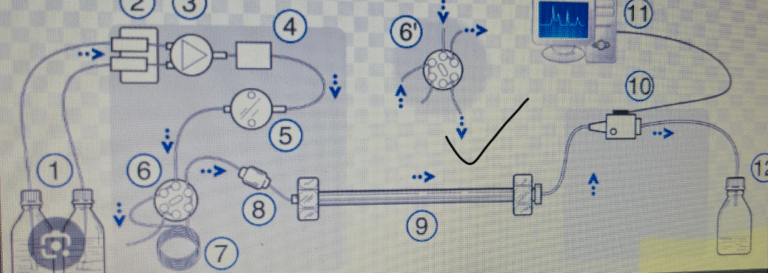
OBJECTIVE To lay down a procedure for maintenance of HPLC column. 2. SCOPE This...
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
OBJECTIVE To lay down a procedure for Evaluation of Stability of standardized Volumetric Solution and to confirm...
1.0 OBJECTIVE To lay down a procedure for maintenance of HPLC column. 2.0 SCOPE This SOP shall...
OBJECTIVE To lay down a procedure for Qualifying the Analyst before assigning them the analytical work. SCOPE...
1-OBJECTIVE To lay down a procedure for product recall. 2-SCOPE This procedure is applicable for Product recall...
Purpose: To define the procedure for handling of market complaints. Scope: This guideline is...