In Pharmaceutical Industries human error and its reduction is very bib challenge. During regulatory audit most of...
Investigation
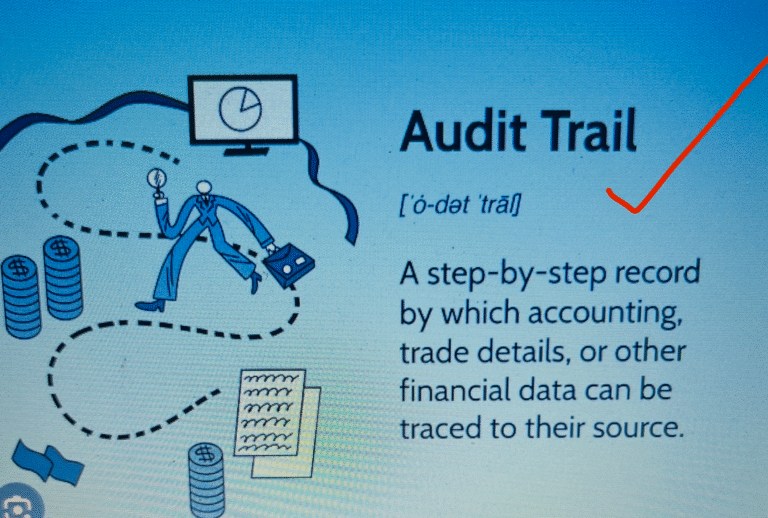
Audit Trail: “An audit trail (also called audit log) is a security-relevant chronological record, set of records, and/or destination and source...
1.0 OBJECTIVE: To lay down a procedure for investigation and evaluation of out-of-specification test results obtained...
Root cause analysis is a systematic process for identifying the true reason for a problem, not just...
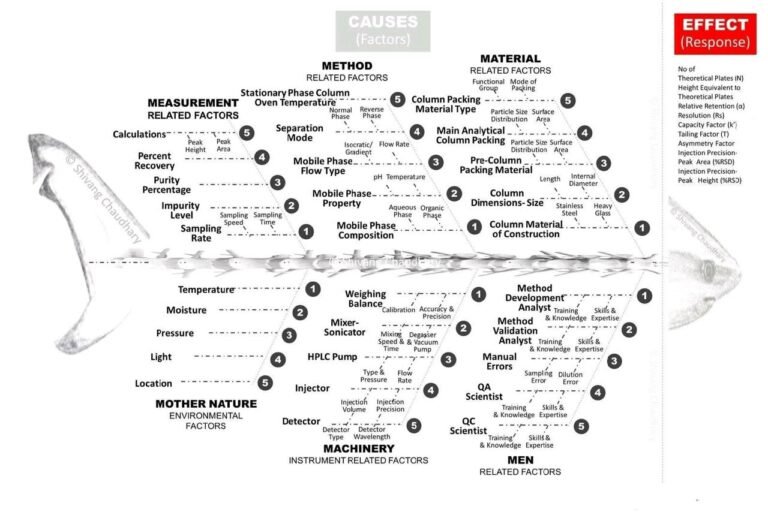
In Pharmaceutical Industries generally following investigation tools are used by investigator- Different Investigation tools: A-5W and 1-How:...
Form FDA 483: USFDA declare an audit of any Pharmaceutical plant after ANDA filling or schedule GMP...
Many Pharmaceuticals organization received 483 due to selection of improper investigation tools. For example, system suitability is...
In pharmaceutical industries specially in Quality control laboratory, it is very difficult to decide that the event...
In Pharmaceutical Industries, in most of the cases investigator has fixed many CAPAs without identifying the actual...
In Pharmaceutical Industries, Lab Incident investigation and root cause analysis to identify the source of Extraneous peak...