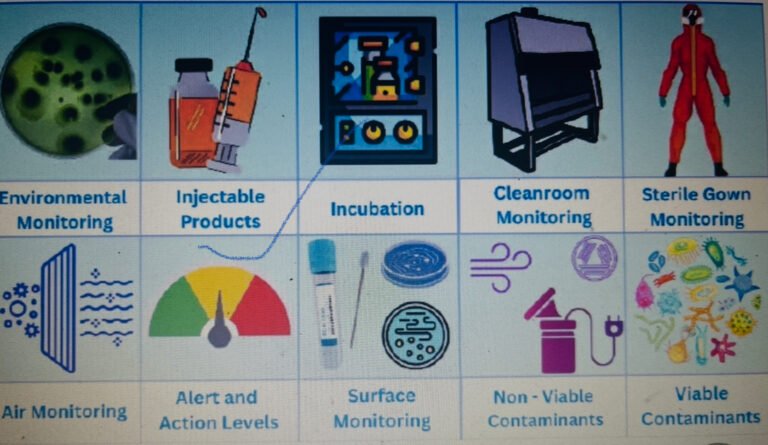
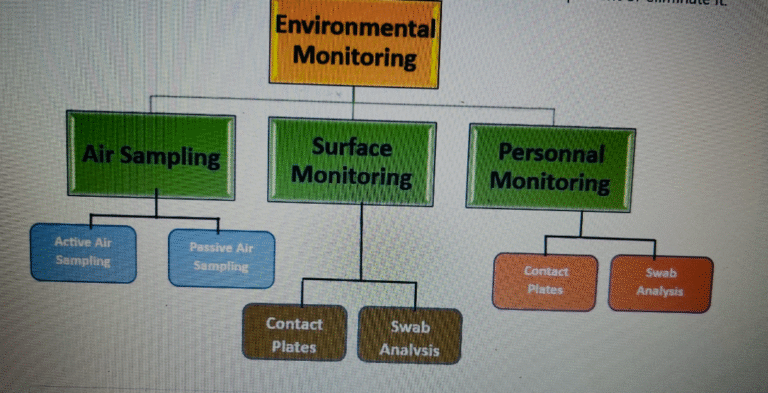
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
Mfg
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
1-OBJECTIVE To lay down a procedure for product recall. 2-SCOPE This procedure is applicable for Product recall...
Cleaning Validation Manufacturing Facility & equipment – Manufacturing facility is nothing different, the same principles apply....
In Pharmaceutical Industries human error and its reduction is very bib challenge. During regulatory audit most of...
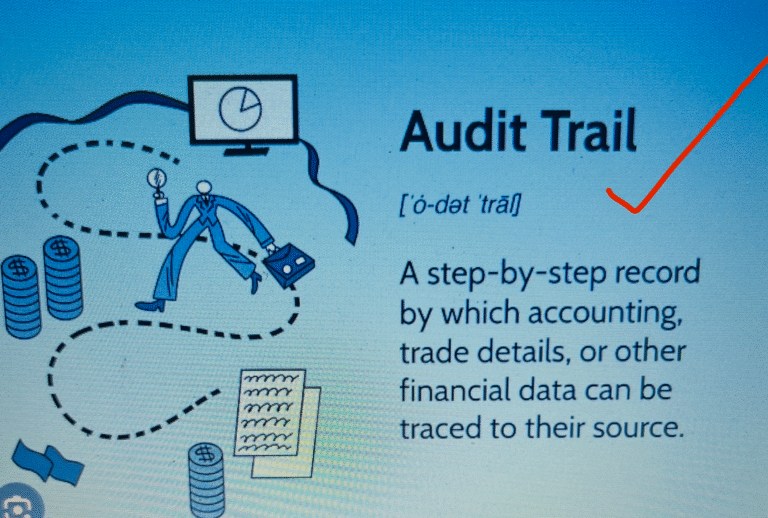
Audit Trail: “An audit trail (also called audit log) is a security-relevant chronological record, set of records, and/or destination and source...
Introduction: Cleaning Validation is very important topic in Pharmaceutical Industries. Our cleaning procedure and cleaning validation should...
Brief: In any pharmaceutical organization compliance of 21 CFR part 11 is very important and basic requirements...
1.0 OBJECTIVE: To lay down a procedure for investigation and evaluation of out-of-specification test results obtained...
In Pharmaceutical Industries, it’s is very important to know the guidance for significant figure and rounding of...