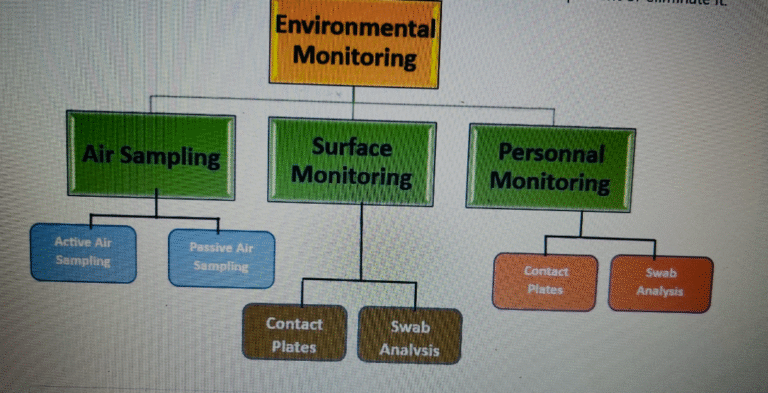
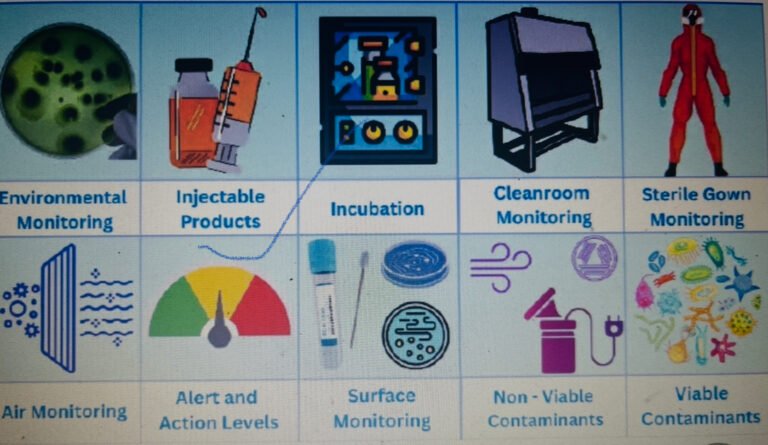
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
QA
1-OBJECTIVE To lay down a procedure for product recall. 2-SCOPE This procedure is applicable for Product recall...
Purpose: To define the procedure for handling of market complaints. Scope: This guideline is...
Cleaning Validation Manufacturing Facility & equipment – Manufacturing facility is nothing different, the same principles apply....
In Pharmaceutical Industries human error and its reduction is very bib challenge. During regulatory audit most of...
Transit (Transportation ) stability study is very important for Pharmaceutical Industries to export the medicines to other countries. After ...
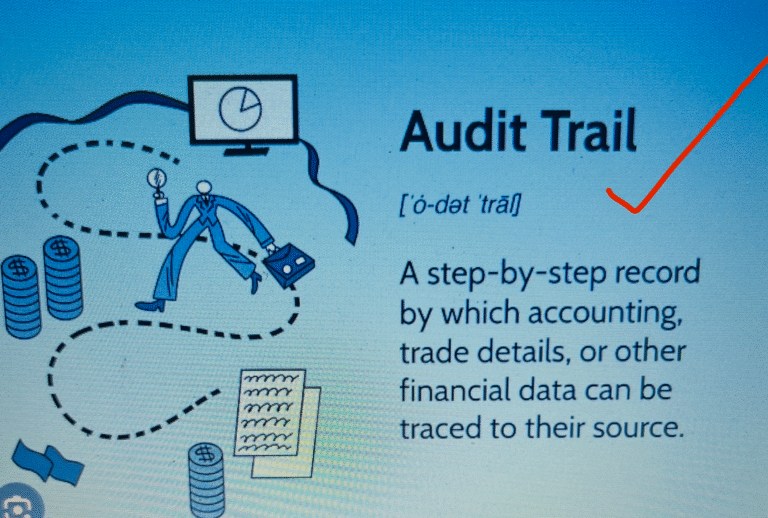
Audit Trail: “An audit trail (also called audit log) is a security-relevant chronological record, set of records, and/or destination and source...
Introduction: Cleaning Validation is very important topic in Pharmaceutical Industries. Our cleaning procedure and cleaning validation should...
Brief: In any pharmaceutical organization compliance of 21 CFR part 11 is very important and basic requirements...
As per ICH “significant change” the changes occur in the drug product during the stability study in Accelerated condition (ACC). In...