1. OBJECTIVE To lay down a procedure for preparation, storage and use of culture media for...
Quality Control
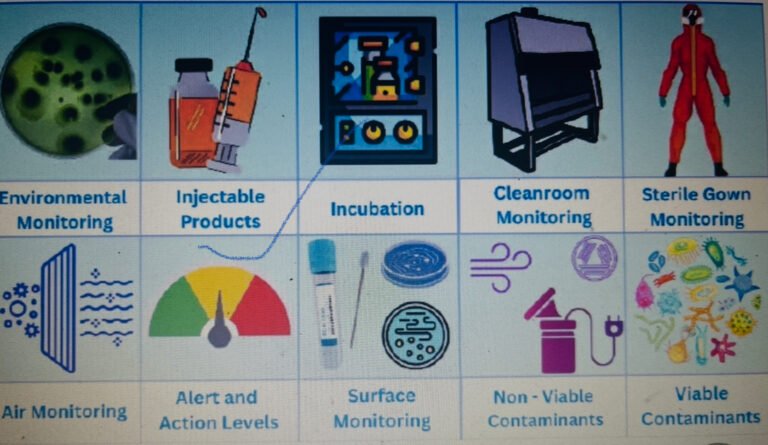
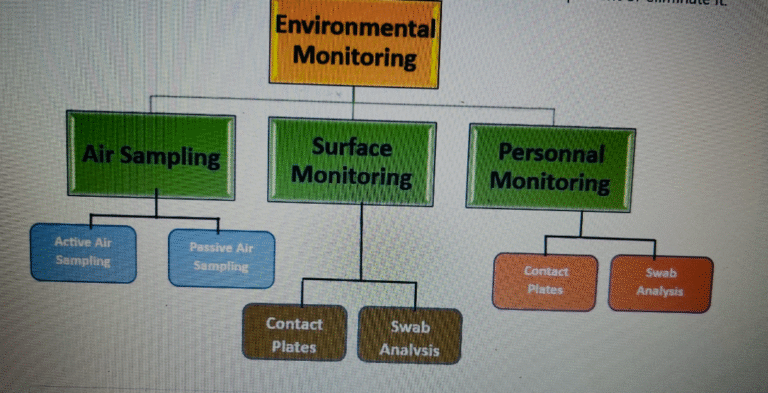
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
OBJECTIVE To lay down a procedure to provide general guideline to be followed in Quality control department...
OBJECTIVE To lay down a procedure for maintenance of HPLC column. 2. SCOPE This...
OBJECTIVE To lay down a procedure for Environmental Monitoring of Manufacturing Area. 2. SCOPE...
OBJECTIVE To lay down a procedure for Evaluation of Stability of standardized Volumetric Solution and to confirm...
1.0 OBJECTIVE To lay down a procedure for maintenance of HPLC column. 2.0 SCOPE This SOP shall...
OBJECTIVE To lay down a procedure for Qualifying the Analyst before assigning them the analytical work. SCOPE...
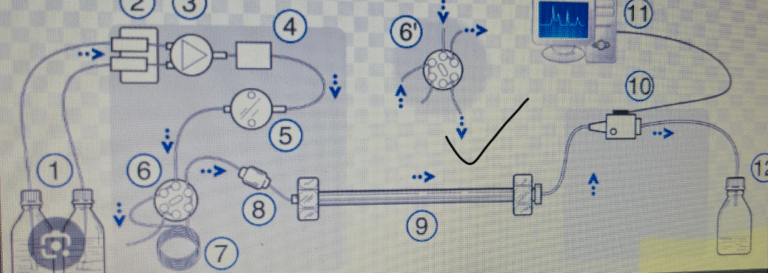
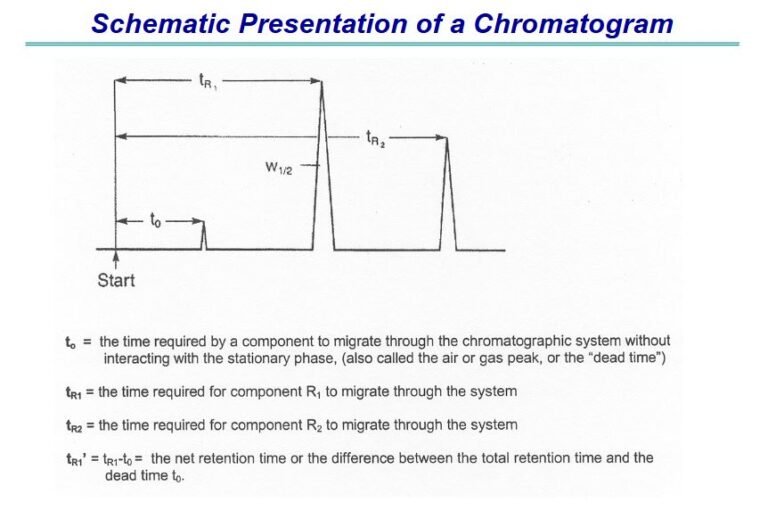
INTRODUCTION Chromatographic separation techniques are multistage separation procedures in which the components of a sample are distributed...